[vc_row][vc_column][vc_column_text]Nuvectra Corporation is a neurostimulation company that focuses on helping physicians to improve the lives of people with chronic neurological conditions. The Company’s Algovita Spinal Cord Stimulation (SCS) System (Algovita) is the Company’s commercial offering and is Conformite Europeene (CE) marked and the United States Food and Drug Administration (FDA) approved for the treatment of chronic pain of the trunk and/or limbs. Its technology platform also has capabilities under development to support other neurological indications, such as sacral nerve stimulation (SNS) and deep brain stimulation (DBS). In addition, its NeuroNexus Technologies, Inc. (NeuroNexus) subsidiary designs, manufactures and markets neural-interface technologies for the neuroscience clinical research market. Its Virtis is an application of the Company’s neurostimulation technology platform and its first product for the SNS market. Its subsidiaries include Algostim, LLC (Algostim) and PelviStim LLC (PelviStim).
Check out our last patent-backed bankruptcy report: TinTri Inc.
Patent-Backed Bankruptcy Report Archive[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][vc_empty_space][vc_tta_tabs][vc_tta_section title=”Company Info” tab_id=”1485966886423-b3803678-c7ec5ac5-4518fd03-b29a”][vc_column_text]
|
Address 5830 Granite Pkwy Ste 1100 Plano TX 75024 |
Ownership
Public (NVTRQ) Industry Surgical and Medical Devices |
[/vc_column_text][/vc_tta_section][vc_tta_section title=”Patent-Backed Value Estimation ” tab_id=”1485966886486-c8105d58-76bb5ac5-4518fd03-b29a”][vc_column_text]Nuvectra Corp Debt-Asset Leverage Estimate
| Chapter Type | Case Number | Industry/Description | ||
| 11 | 19-43090 | Medical Devices | ||
| Portfolio Valuation Range | Asset Valuation Range | Total Assets Valuation Range | Liability Range | Leverage Ratio Range |
| $2.5 – 11M | $10-50M | $12.5 – 60M | $10-50M | 1.2 – 1.25 |
Patent Portfolio
|
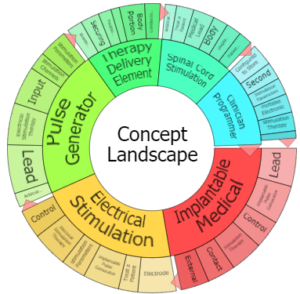 |
[/vc_column_text][/vc_tta_section][vc_tta_section title=”Featured Assets” tab_id=”1485967825251-b275f45c-d8b55ac5-4518fd03-b29a”][vc_column_text]Nuvectra Corp. Portfolio Summary
-
US10471265 B2
A anchor for an implantable medical device includes an anchor body and a locking member. The anchor body includes a first trough extending along a first axis. The locking member is coupled to the anchor body and rotates with respect to a second axis, between an unlocked position and a locked position. The locking member includes protruding members that define a second trough aligned with the first trough when the locking member is rotated to the unlocked position, so as to form an open path for the implantable medical device to move through the first and second troughs. When the locking member is rotated to the locked position, the protruding members block at least a portion of the first trough to define a tortuous path between the first trough and the second trough so as to restrict a movement of the implantable medical device through the first and second troughs.
-
US9068587 B2
In various examples, a set screw apparatus includes a set screw including a set screw body. An abutment extends radially outwardly from the set screw body and longitudinally separated from a threaded set screw area of a first length by an unthreaded set screw area of a second length of the set screw body. A block includes a bore. A threaded block area includes a third length, wherein the abutment inhibits movement of the set screw past the threaded block area and removal of the set screw from the bore. The third length is shorter than the second length so that an entirety of the threaded block area can be disposed within the unthreaded set screw area, such that the set screw is freely rotatable within the bore, but retained within the bore, if turned in a first direction with respect to the block.
-
US8868199 B2
The present disclosure involves a method of data-reducing and storing a sensation map. A sensation map associated with a patient is provided. The sensation map includes a graphical depiction of a sensation experienced by the patient. The sensation may be pain or paresthesia experienced by the patient in response to an electrical stimulation therapy. A data file is generated. The data file has a data size less than a data size of the sensation map. The data file contains digital information allowing a reconstruction of the sensation map. Electronic communication is then established with an implanted medical device located inside the patient’s body. Thereafter, the data file is sent to the implanted medical device for storage. The stored data files are retrievable by another clinician programmer later to reconstruct the sensation map.
[/vc_column_text][/vc_tta_section][/vc_tta_tabs][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column width=”1/4″][vc_cta h2=”Portfolio Valuation ” h4=”IPVal offers portfolio valuation services.” add_button=”right” btn_title=”Contact Us ” btn_add_icon=”true” btn_link=”url:http%3A%2F%2Fipval.com%2Fcontact-us%2F%231467647561464-2a34809f-529c||”][/vc_cta][/vc_column][vc_column width=”3/4″][contact-form-7 id=”599″][/vc_column][/vc_row][vc_row][vc_column][/vc_column][/vc_row]